After Opioid Overdose Emergency, Few Patients Receive Timely Follow-up
Easing regulatory restrictions due to Covid-19 may provide solutions
This post originally appeared on the Penn LDI Health Policy$ense blog.
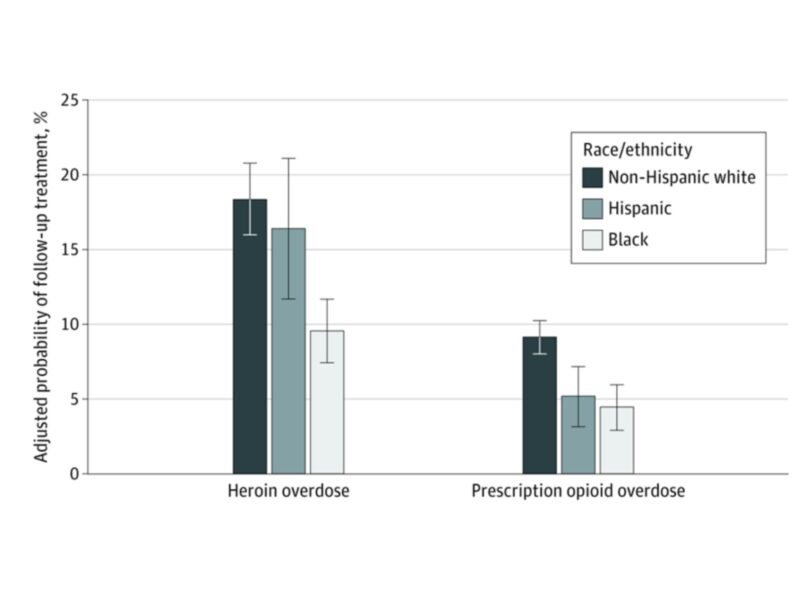
An opioid overdose is significantly more than an isolated event. Patients who present to the emergency department (ED) with overdose have a 6 percent risk of dying in the following year. As with other high-risk acute conditions, we expect patients who survive overdose to receive evidence-based treatment after leaving the hospital. Whether the overdose was due to prescription opioids or injection drugs, the first occurrence or recurrent, in people with diagnosed opioid use disorder (OUD) or not – we know that timely follow-up care can save lives. But our recent national study showed that just 16% of privately insured patients obtain that essential care.
We reviewed commercial insurance claims for about 6,500 patients who presented to the ED with opioid overdose between 2011 and 2016. Only 1 in 6 obtained follow-up treatment in the 90 days after the overdose – including medication treatment, outpatient clinic visits, or rehabilitation services. For the majority of patients who had not previously received treatment for opioid use disorder (OUD), only 1 in 10 obtained follow-up.
As shown, we found a striking racial disparity as well: black patients were half as likely to access treatment as non-Hispanic white patients.
Our study amplifies the critical question facing policymakers, providers, and patients – how do we increase access to OUD treatment, particularly for patients during the vulnerable time after an overdose?
Pandemic policy changes
The Covid-19 pandemic has made timely access to OUD treatment even harder, while the social and economic effects of the pandemic are likely to exacerbate the still-raging opioid epidemic. EDs around the county have reported dramatic reductions in volumes for all patients, including those with heart attack and stroke. Opioid overdose is no different. More patients may experience an overdose in social isolation without bystander rescue and calling emergency medical services. More fatal overdoses at home represent a tragic missed opportunity to engage patients in treatment in the ED.
However, the pandemic has also spurred policy changes that may point to novel solutions. On March 7, the Drug Enforcement Agency (DEA) permitted initiation of buprenorphine through telemedicine visits, without the previously required in-person consultation and exam. Buprenorphine is an effective treatment to prevent relapse and repeat overdose. The same day, the Office of Civil Rights waived penalties for Health Insurance Portability and Accountability Act (HIPAA) violations against health care providers for using non-HIPAA compliant technologies, including Facetime and Skype. The DEA subsequently loosened regulations further and allowed prescribers with x-waivers to initiate buprenorphine through audio-only visits. Also in March, SAMHSA allowed states to request waivers to allow opioid treatment programs (OTPs) to provide extended take-home of methadone instead of requiring daily in-person visits.
These regulatory changes have prompted the development of innovative programs around the country to remotely counsel, connect, and initiate treatment for patients with OUD. At Penn Medicine, the Center for Opioid Recovery and Engagement (CORE) provides a telehealth service for buprenorphine as well as direct access to expert Certified Recovery Specialists (CRS) through a newly established phone triage line. Similar programs have been created in cities and states around the country, accelerating previously stalled efforts to expand virtual care options OUD medications.
Is telehealth the solution to expanding access OUD treatment? The pandemic may provide us the opportunity to answer this essential question. We don’t yet know whether providers will widely adopt telehealth practices, whether payers will continue to reimburse this delivery model, and most importantly – whether patients are able to engage and sustain treatment without in-person care.
Answering these questions may take some time, but our study has an important implication for policymakers. Access to treatment in the opioid epidemic was already at critically low levels when the pandemic hit. The crisis precipitated immediate and important policy changes that should become permanent, especially since the social and economic effects of the pandemic are likely to worsen the still-raging opioid epidemic.
The shift to telemedicine, and the pandemic in general, may have many unintended consequences for OUD treatment. For example, many practices may be using telemedicine for established patients but may be reluctant to accept new patients. Patients may be directed to buprenorphine treatment although methadone remains an effective, and sometimes preferred, treatment modality.
Finally, the shift to telemedicine may in fact worsen the racial disparities that we described in our study. Vulnerable populations and racial minorities are known to experience disparities in access to telehealth, with less access to technology and technological literacy. As telehealth continues to expand for patients with OUD, we must ensure that all populations have equitable access to these novel treatment pathways. COVID-19 has demonstrated gaping vulnerabilities of marginalized populations and racial minorities. We cannot afford to have our innovative solutions to the crisis to create an even more inequitable system of care.
The article, “Incidence of Treatment for Opioid Use Disorder Following Nonfatal Overdose in Commercially Insured Patients” was published in JAMA Network Open on May 27, 2020. Authors include Austin S. Kilaru, MD, MSHP; Aria Xiong, MS; Margaret Lowenstein, MD, MPhil; Zachary F. Meisel, MD, MPH, MSHP; Jeanmarie Perrone, MD; Utsha Khatri, MD; Nandita Mitra, PhD; and M. Kit Delgado, MD, MS.
This study was supported by a Penn LDI pilot grant to Dr. Kilaru. Dr. Kilaru was awarded a Pilot Grant from CHERISH, and Drs. Lowenstein and Delgado are previous CHERISH Pilot Grant Recipients.