State Medicaid Spending on Sovaldi: Wide Variation in Spending and Utilization in 2014
With a price tag of $1,000 per pill and $84,000 for a 12-week course of Sovaldi (sofosbuvir), Gilead Sciences prompted widespread concern about whether its new treatment for hepatitis C (HCV) would bankrupt public and private payers. These concerns were particularly significant for state Medicaid programs, which face both limited state budgets and high HCV prevalence among beneficiaries. Today in the New England Journal of Medicine, LDI Fellow Joshua Liao and colleagues describe early Sovaldi utilization patterns by Medicaid programs in 2014, the first full year after its approval, and report widespread variation across states.
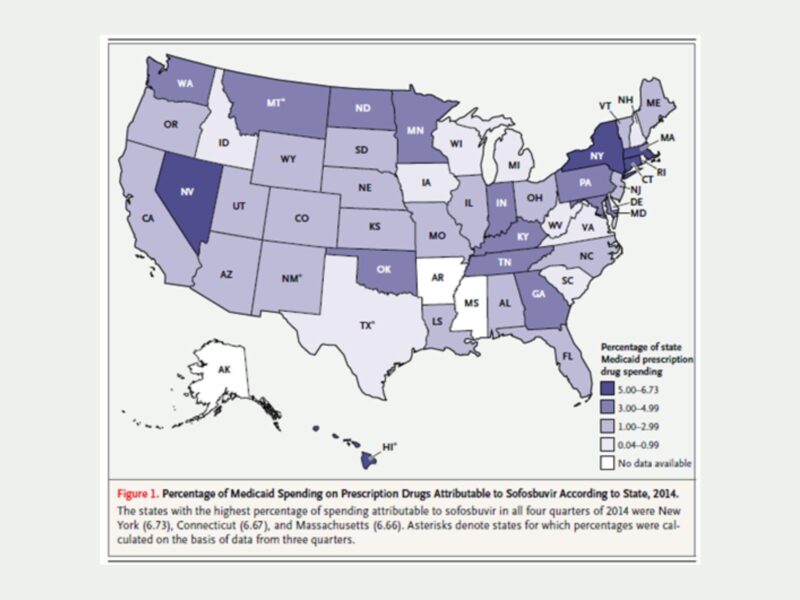
The authors found that overall, Medicaid programs spent more than $1.3 billion on Sovaldi in 2014. Individual states spent anywhere from 0.5% to 6.7% of their prescription drug budgets on Sovaldi, as the map below shows. States that expanded Medicaid under the ACA spent a higher proportion of their budgets on Sovaldi than those that did not expand coverage.
To control costs, states implemented various restrictions on which HCV patients are eligible to receive the drug, a finding reflected in the substantial state program-level variation in Sovaldi utilization observed by the study authors. In 2014, the drug accounted for anywhere from 2% (Texas) to 44% (Hawaii) of all HCV-related prescriptions, although utilization did not correlated with state-level incidence of HCV infection.
As the first big-picture analysis of state Medicaid program spending on Sovaldi — which along with other new HCV medications led to historic surges in drug spending in 2014 — Liao and colleagues’ study highlights the need for policies to guide state decisions. As they conclude:
These results — together with Medicaid budgetary constraints, high drug costs, and the increasing availability of other expensive, new medications — underscore the need to identify effective strategies to guide policy and reimbursement in order to ensure parity and appropriateness in the use of sofosbuvir among the Medicaid population and other high-need populations, such as prisoners and low-income patients who are not eligible for Medicaid, especially in states that did not expand Medicaid coverage.