Cost-Effectiveness of Fibrosis Staging
A microsimulation study of clinical- and cost-effectiveness of liver disease staging in hepatitis C virus infection

More than 4 million people in the U.S. live with a hepatitis C virus (HCV) diagnosis.
However, as of 2022, fewer than one-third of individuals infected with HCV have been cured due to barriers related to high direct-acting antiviral (DAA) pricing and the steps involved in determining someone’s degree of liver disease or fibrosis.
In a new study, lead author and CHERISH Research Affiliate Rachel Epstein compared common fibrosis staging strategies – tests to determine the degree of liver damage someone has – for clinical outcomes and cost-effectiveness to better understand the guidelines for liver disease assessment for HCV. These staging assessments are a key aspect of HCV infection pre-treatment evaluation, but guidelines do not agree on a single testing modality due to trade-offs in availability and accuracy. The five staging strategies included in the study:
- No staging or treatment
- Indirect Fibrosis-4 (FIB-4) serum biomarker testing only
- Transient elastography (TE; FibroScan™) only
- Staged approach-
- FIB-4 for all
- TE for intermediate FIB-4 score (1.45-3.25)
- Both tests (FIB-4 and TE) for all
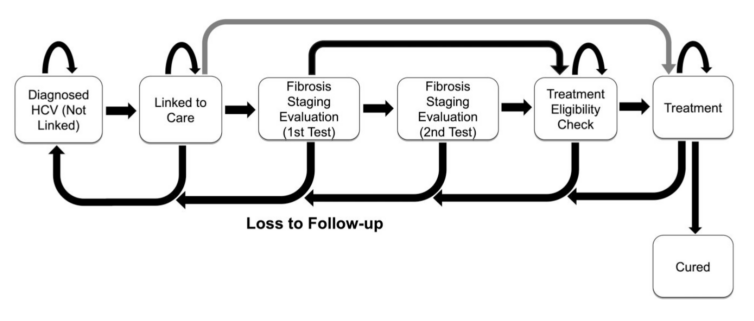
The Hepatitis C Cost-Effectiveness (HEP-CE) model used in this study simulated 10 million adults with chronic HCV receiving care at federally qualified health centers in the U.S. through a lifetime microsimulation. Individuals in the model move through a series of health and disease states, medical interventions, and death. The study cohort can be tested for HCV, diagnosed, and linked to care. This linkage could involve either fibrosis staging, HCV treatment and cure, or loss to follow up (LTFU). Outcomes Epstein and co-authors reviewed included infections cured, cirrhosis cases, liver-related deaths, costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratio (ICERs).
Epstein and co-authors projected that staging with just FIB-4 predicted the best clinical outcomes with 88% cured, 9% developing cirrhosis, and 5% having liver-related deaths. Testing with TE only presented worse clinical outcomes with 59-77% cured, 17-30% developing cirrhosis, and 12-23% having liver-related deaths. All TE strategies had higher costs per QALY and were only cost-effective when there was no LTFU in the process of obtaining the TE. In the scenario where no testing is available without incurring LTFU (as applies in certain clinical settings such as mobile testing units and treatment in clinics without on-site blood draw capabilities), no staging was actually the most clinically and cost-effective strategy.

Testing with FIB-4 projected an average remaining life expectancy of nearly 31 years – a gain of almost 8 years over the comparator strategy of no staging and no treatment. Discounted costs and QALYs were also highest in FIB-4 only, meaning this strategy is the most cost-effective and lifesaving in the long-term. Most one-way and probabilistic sensitivity analysis yielded similar comparisons to the base case scenario, demonstrating findings were robust to varied parameter values. LTFU was the major driver of worse clinical outcomes – only when no LTFU occurred in TE strategies did they become cost-effective. This indicates that starting HCV treatment before a patient is LTFU is the most important and effective factor for predicting the best clinical outcomes.
FIB-4 staging alone was found to have the most optimal outcomes and be the most cost-effective comparatively. It is important to treat as soon as possible – even if that means treating without any staging – to limit loss to follow-up and subsequent progression of liver disease.
Key Findings
- Starting HCV treatment before a patient is LTFU is the key factor in predicting the best clinical outcomes.
- If no staging is available on site, test-and-treat without staging is the most clinically and cost-effective strategy.
It is critical that clinicians, policymakers, and payers understand the consequences of requiring TE or other staging prior to HCV treatment. Patients may be LTFU and develop worsening liver disease before receiving HCV treatment. When patients were LTFU even 5% of the time in the TE strategy, there were 23% more cirrhosis diagnoses and 38% more liver-related deaths in comparison to FIB-4 only. The authors urge clinicians to not wait to treat HCV in favor of a slightly improved assessment of a patient’s cirrhosis status.
The study, “Clinical- and Cost-Effectiveness of Liver Disease Staging in Hepatitis C Virus Infection: A Microsimulation Study,” was published in Clinical Infectious Diseases on November 13, 2024.