
Health Economic Outcomes of a Minimal Monitoring Approach to Providing HCV Therapy
In a new article in Hepatology Communications, lead author and CHERISH Population Data & Modeling Core Director Benjamin Linas and colleagues report new economic analyses from MINMON, an AIDS Clinical Trial Group (ACTG 5360) study that used a minimal monitoring approach to provide treatment for chronic hepatitis C virus (HCV). Findings suggest that the simplified […]

Hepatitis C Virus Screening in Pregnant and Nonpregnant Women After Universal Screening Guidelines
Hepatitis C virus (HCV) testing guidelines were updated in 2020 to provide universal, one-time screening for adults ages 18 through 79 and during each pregnancy. This departure from their 2013 recommendation not only aims to improve early detection of HCV across age groups but also acknowledges pregnancy as a critical period for diagnosing and treating […]

Cost-Effectiveness of Fibrosis Staging
More than 4 million people in the U.S. live with a hepatitis C virus (HCV) diagnosis. However, as of 2022, fewer than one-third of individuals infected with HCV have been cured due to barriers related to high direct-acting antiviral (DAA) pricing and the steps involved in determining someone’s degree of liver disease or fibrosis. In […]

Racial and Ethnic Disparities Along the Hepatitis C Care Cascade Among Priority Populations
Over the last two decades, injection opioid use has increased among reproductive-aged women, with resultant increases in mother-to-child hepatitis C virus (HCV) transmissions in the U.S. Direct-acting antiviral (DAA) treatment is highly effective at reducing and eliminating the risk of HCV transmissions. Yet, studies show lower odds of receiving DAA treatment for women compared to […]

Announcing the 10th Pilot Grant Cohort Advancing the Treatment of Substance Use Disorders, HCV, and HIV
CHERISH is excited to welcome a new pilot grant cohort for the 10th year. Spearheaded by CHERISH Pilot Grant Program Director Brandon Aden, the program is designed to help researchers gain familiarity with health economic evaluations, apply related methodologies to their pilot research, and build out the next stage of their careers. This year, CHERISH […]
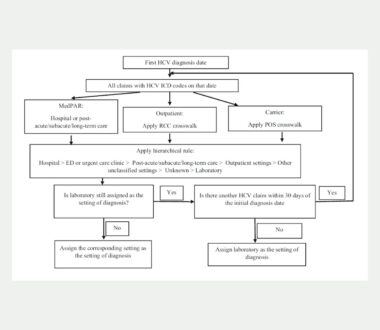
Association between claims-based setting of diagnosis and treatment initiation among Medicare patients with Hepatitis C
Direct-acting antivirals (DAAs) have been available since 2015 for hepatitis C (HCV). Nonetheless, very few patients with HCV initiate DAA treatment, ranging from 5% to 30%. The location where a patient is first diagnosed can also influence the care patients receive afterward, especially when a referral to a specialist or coordinated care is needed. In […]
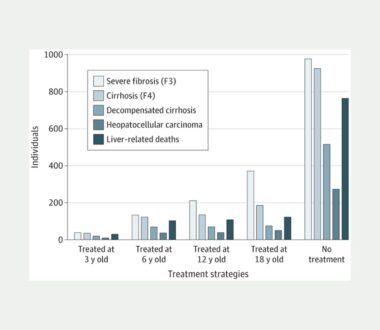
Cost-Effectiveness of Strategies for Treatment Timing for Perinatally Acquired Hepatitis C Virus
Children with perinatally acquired hepatitis C virus (HCV) are now eligible to receive direct-acting antivirals (DAAs), a highly effective treatment for HCV, as early as the age of 3. Despite existing studies demonstrating DAA’s high efficacy and tolerance among adults and children between the 3 and 12 years old, there has been low uptake due […]
Initiation of Hepatitis C Treatment Low Among Medicaid Recipients
While there are highly effective treatments for the hepatitis C virus (HCV), only 1 in 5 Medicaid enrollees diagnosed with HCV started treatment, according to a retrospective study led by researchers at Weill Cornell Medicine and Cornell University’s Ithaca campus. The findings revealed that treatment uptake rates were even lower among people under 30, women, […]

Intern Spotlight: Valeria Arango, Benicio Beatty, Ameya Komaragiri, Carlos Ponce de Leon Mendez, and Ella Salim
During the peak hour of a July summer day, CHERISH colleagues and interns from Boston Medical Center logged onto Zoom while investigators and staff in New York City filled a conference room located on the Upper East Side at Weill Cornell Medicine. Three minutes past twelve, Caroline Savitsky, program manager in the Section of Infectious […]

CHERISH Welcomes Pilot Grant Recipients to Advance the Treatment of Substance Use Disorders, HCV, and HIV
Meet the ninth cohort of early-career investigators from the states of California, Texas, and Washington. CHERISH awarded each investigator up to $20,000 for a pilot study that focuses on conducting health economics research in substance use, hepatitis C virus (HCV), and HIV. This year, CHERISH also encouraged applicants to integrate drug overdose prevention, the syndemics […]

Catching Up with Pilot Grant Recipient: Rachel Epstein
Rachel Epstein is a clinician-scientist with demonstrated experience in analyzing large datasets to inform hepatitis C (HCV), HIV, and substance use disorder interventions. A CHERISH pilot grantee in 2019, she worked closely with her co-investigators Benjamin Linas, CHERISH Population Data and Modeling Core director, and Shashi Kapadia, a CHERISH Research Affiliate and an infectious diseases physician at Weill […]

Five Researchers Receive CHERISH Pilot Awards to Advance the Treatment of Substance Use Disorders, HCV, and HIV
The Center for Health Economics of Treatment Interventions for Substance Use Disorder, HCV, and HIV (CHERISH) is proud to recognize five early-stage researchers who will receive up to $20,000 in pilot grant funding between 2022 and 2023. Through the CHERISH pilot grant, researchers can investigate innovative methodologies or applications, collect preliminary data to inform external […]
Engage with CHERISH
Submit a Consultation Request or Contact Us to learn more about how CHERISH can support your research or policy goals.