Hepatitis C Virus Screening in Pregnant and Nonpregnant Women After Universal Screening Guidelines
Study highlights increase in HCV screening rates among pregnant women after guidelines were updated but adherence to guidelines was still less than 40%.

Hepatitis C virus (HCV) testing guidelines were updated in 2020 to provide universal, one-time screening for adults ages 18 through 79 and during each pregnancy. This departure from their 2013 recommendation not only aims to improve early detection of HCV across age groups but also acknowledges pregnancy as a critical period for diagnosing and treating HCV.
Researchers from Boston Medical Center and Boston University, including lead author and infectious diseases fellow Roshni Singh, senior author and CHERISH Research Affiliate Rachel Epstein, CHERISH pilot grant recipient Breanne Biondi, and CHERISH Population Data & Modeling Core Director Benjamin Linas, published a research letter in JAMA to further explore the impact of the updated HCV screening guidelines on pregnant and nonpregnant women. The team used the TriNetX database to examine HCV screening rates among over 13 million patients between 2014 to 2022 from 68 U.S. healthcare organizations. HIV testing rates were used as a negative control group as HIV screening guidelines remain unchanged.
Key Findings
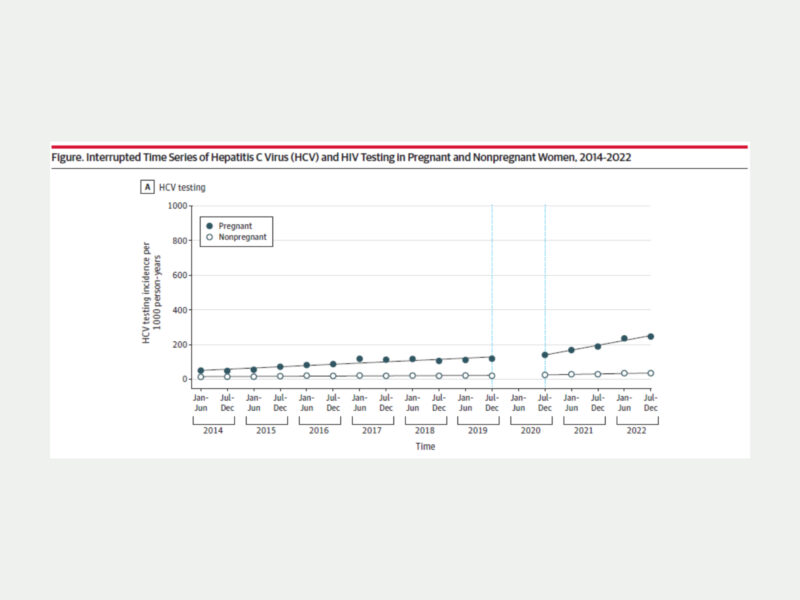
Following the updated guidelines, researchers found a statistically significant increase in the HCV screening rate—by 21 HCV tests per 1,000 person-years in each 6-month interval—for pregnant women compared to women who were not pregnant.
- After the updated guidelines were implemented in 2020, HCV screening per 1,000 person-years among pregnant women nearly doubled from 141 to 253 tests.
- Comparatively, among nonpregnant women, HCV screening per 1,000 person-years during the same period increased from 29 to 37 tests.
- There was no increase in screening for HIV among pregnant or nonpregnant women during the same period
- By December 2022, 39% of pregnant women and 9% of nonpregnant women had received an HCV test. In comparison, 90% of pregnant women had received an HIV test.
While findings reveal an increase in testing rates after the 2020 guidance update, the percentage of pregnant women and nonpregnant women who received an HCV test by the end of the study were below guideline recommendations. For that reason, researchers emphasize that future national and novel HCV elimination strategies should focus on prenatal care as a critical intervention period to improve HCV testing.
“Hepatitis C Virus Screening in Pregnant and Nonpregnant Women After Universal Screening Guidelines” was published as a research letter in JAMA on February 25, 2025. This study has been featured in Healio, HCP Live, and MedScape.