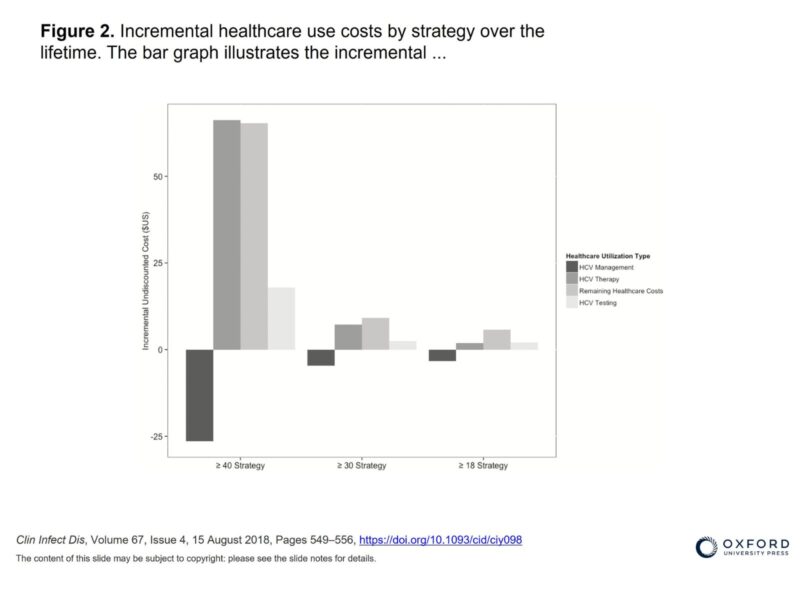
The USPSTF is responsible for developing clinical practice guidelines for prevention and screening based on a careful review of the quality and strength of existing evidence including benefits and costs. In the draft recommendation the USPSTF cites a modeling study by CHERISH Research Affiliate Joshua Barocas, MD and CHERISH HCV and HIV Core Director Benjamin Linas, MD, MPH from Boston Medical Center, and colleagues from CDC, Massachusetts Department of Public Health, and Stanford. Dr. Barocas’ study used a Monte Carlo simulation model to compare the current USPSTF guidelines for birth cohort screening to different strategies that expand screening to younger populations with increased incidence. The study estimated that compared to previously recommended birth cohort screening, expanded screening to adults 18 or older would identify 256,000 additional cases of HCV infection and lead to 280,000 additional cures and 4,400 fewer cases of hepatocellular carcinoma over the cohort lifetime with an incremental cost effectiveness ratio of $28,193/ QALY in 2016 US dollars and an overall increased life expectancy among those with HCV.
The draft evidence review supporting this new recommendation prepared by the Agency for Health Care Research & Quality cites other modeling studies in addition to the one authored by Drs. Barocas and Linas. These include another study examining the cost-effectiveness universal screening, a study authored by CHERISH Research Affiliate Sabrina Assoumou, MD, MPH of Boston Medical Center and colleagues, including Dr. Linas and Dr. Bruce Schackman and Jared Leff from Weill Cornell Medicine, examining the cost-effectiveness of one-time HCV screening for adolescents and young adults in primary care settings, and two studies examining HCV screening in prenatal care.
Similar to Dr. Barocas’ study, Eckman et al estimated the cost-effectiveness of universal one time HCV screening for all adults 18 and older compared with the current birth cohort screening guidelines using a Markov state transition model. The study found that universal one-time HCV screening and treatment for all adults was cost-effective even at very low rates (0.07%) of HCV prevalence in the population compared with no screening or current birth-cohort screening guidelines. CHERISH Research Affiliate Dr. Assoumou’s study focused on the cost-effectiveness of one time testing among young adults and adolescents aged 15-30 years old, which includes individuals who are younger than those covered by the USPSTF recommendation under review for one-time screening in adults 18 years or older. Dr. Assoumou’s study examined different screening methods and care delivery strategies within an urban community healthcare setting, and found potential cost and effectiveness differences by provider type and screening diagnostic method (rapid assessment or venipuncture).
HCV prevalence among pregnant women in the US doubled from 2009-2014, making screening and diagnosis of HCV among pregnant women a critical opportunity for diagnosis of the mother and of children infected with HCV through vertical transmission. Two studies cited in the draft evidence review also examined HCV screening among pregnant women in prenatal care. Chaillon et al used a Markov model to examine the cost-effectiveness of universal prenatal HCV screening followed by postnatal treatment compared to background risk-based screening and found universal prenatal HCV screening among pregnant women was cost effective. Similarly, an analysis by Abriana Tasillo, CHERISH Investigator Benjamin Linas, and colleagues evaluated the cost-effectiveness of one-time prenatal screening and postnatal treatment during pregnancy compared to current practice that does not require screening. This analysis demonstrated that prenatal screening increased identification of neonates exposed to HCV at birth by 48%, increased the proportion of cases achieving SVR within 5 years by 11%, and consequently reduced HCV attributable mortality by 16%. Together, these findings the USPSTF recommendation for prenatal screening and the potential treatment benefits of both mother and child.
In November 2019 the Centers for Disease Control and Prevention (CDC) announced it was seeking comments on new hepatitis C screening recommendations that also recommends screening at least once in a lifetime for all adults 18 or older, as well as screening for all pregnant women, in addition to risk-based screening. The CDC recommendation relies on much of the same cost-effectiveness literature cited in the USPSTF testing recommendations. The CDC stipulates that its recommendations apply to states and settings where the prevalence of hepatitis C is greater than 0.1% of the adult population, based in part on its review of cost-effectiveness literature which demonstrated that incremental cost-effectiveness ratios were sensitive to HCV prevalence. Currently, there is no state with a hepatitis C prevalence below 0.1%, and only 3 states have an HCV prevalence less than 0.1% among pregnant women according to state birth certificate data. The comment period on the CDC recommendations remains open until December 27. 2019