Cost of Hepatitis C Care Facilitation for HIV/Hepatitis C Co-infected People Who Use Drugs
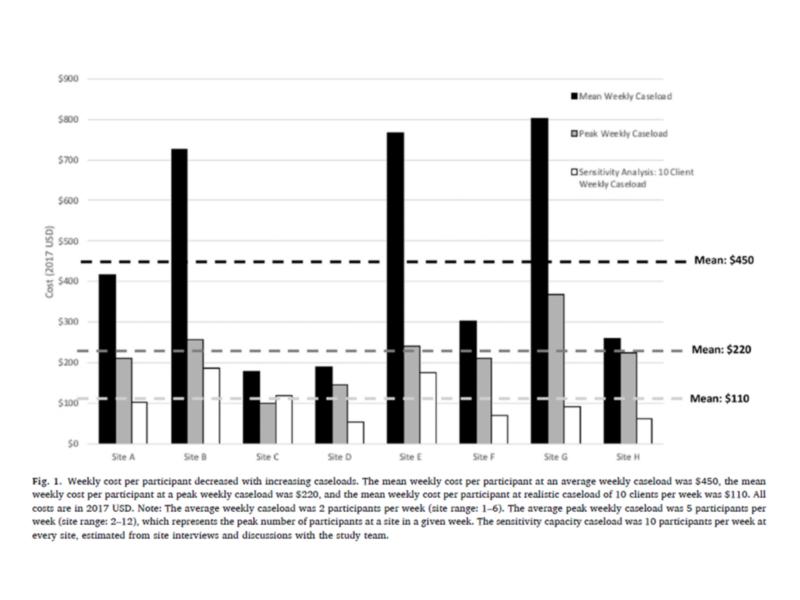
Weekly HCV care facilitation cost per participant decreased with increasing caseloads.
The development of direct-acting antivirals transformed hepatitis C virus (HCV) therapy, allowing patients to experience fewer side effects and non-specialist providers to prescribe HCV treatment with more ease.
Despite these advances, existing social and structural barriers, such as stigma and accessibility of medical care, restrict access to effective HCV treatment for people who use drugs who are co-infected with HIV and HCV. To improve access to care for HIV and HCV co-infected people who use drugs, researchers are looking at models that borrow from successful HIV linkage to care interventions.
Results from CTN-0064, a national clinical trial funded by the National Institute on Drug Abuse, showed that a care facilitator intervention helped HIV and HCV co-infected people who inject drugs advance further in their HCV treatment. To help healthcare organizations understand the cost of care facilitation interventions, lead author Sarah Gutkind, CHERISH Research Affiliates Laura Starbird and Daniel Feaster, CHERISH Methodology Co-director Sean Murphy, and CHERISH Director Bruce Schackman used data from CTN-0064 to estimate the costs of implementing and managing this model of care across eight sites in the United States.

Sarah Gutkind, MPH
Pre-doctoral fellow in the Substance Abuse Epidemiology Training Program at Columbia University Mailman School of Public Health.
In this study, Gutkind and colleagues found that the cost per participant decreases with higher caseloads in real-world settings compared to costs incurred in the intervention trial. In a sensitivity analysis, maintaining a site’s peak caseload during the trial (approximately 5 participants per week on average) was found to lower the cost per participant by a half, from $450 to $220 in 2017 USD. When adapting to a real-world setting with a maximum of 10 cases per week, the cost each week per participant lowered by three quarters, from $450 to $110 in 2017 USD. Co-authors also found the average start-up cost for HCV care facilitation to be approximately $6,320 per trial site. Costs could be further reduced by adapting administrative and recruitment processes for real-world settings, such as maximizing outreach activities through site visitations and database search records, reducing the number of supervision meetings, and leveraging local staff and webinars to conduct start-up trainings.
The weekly cost of hepatitis C virus infection (HCV) care facilitation was estimated to be $450 per HIV and HCV co-infected individual recruited from a clinical trial. In real-world settings with higher caseloads, the cost would decrease to approximately $110 per person.